Congrats to Dr. Graybill and Dr. Wade!
They each successfully defended their theses, though the cake may have suggested a different celebration. Congratulations to Dr. Graybill for successfully defending his thesis "Improving Multiplexed RNA Detection Assays By Interfacing Enzymatic Amplification Strategies with Silicon Photonic Microring Resonators.” Congratulations to Dr. Wade, as well. He defended his thesis "Expanding the Toolbox for Personalized Medicine with Silicon Photonic Microring Resonators and Microfluidic Technologies."
Congrats to Steve on his recent publication!
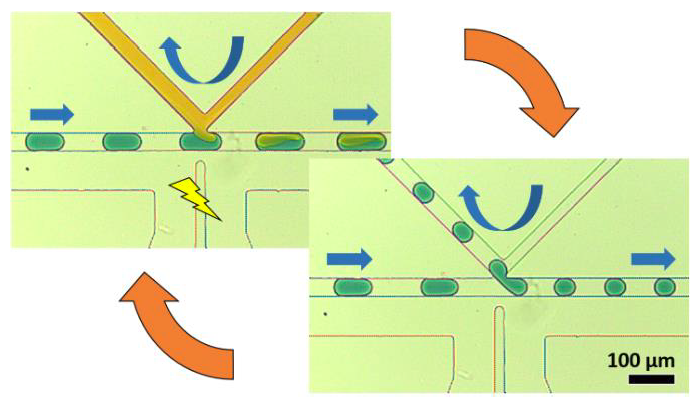
The K-Channel: A Multi-Functional Architecture for Dynamically Re-Configurable Sample Processing in Droplet Microfluidics
ABSTRACT
By rapidly creating libraries of thousands of unique, miniaturized reactors, droplet microfluidics provides a powerful method for automating high-throughput chemical analysis. In order to engineer in-droplet assays, microfluidic devices must add reagents into droplets, remove fluid from droplets, and perform other necessary operations, each typically provided by a unique, specialized geometry. Unfortunately, modifying device performance or changing operations usually requires re-engineering the device among these specialized geometries, a time-consuming and costly process when optimizing in-droplet assays. To address this challenge in implementing droplet chemistry, we have developed the “K-channel,” which couples a cross-channel flow to the segmented droplet flow to enable a range of operations on passing droplets. K-channels perform reagent injection (0-100% of droplet volume), fluid extraction (0-50% of droplet volume), and droplet splitting (1:1-1:5 daughter droplet ratio). Instead of modifying device dimensions or channel configuration, adjusting external conditions, such as applied pressure and electric field, selects the Kchannel process and tunes its magnitude. Finally, interfacing a device-embedded magnet allows selective capture of 96% of dropletencapsulated superparamagnetic beads during 1:1 droplet splitting events at ~400 Hz. Addition of a second K-channel for injection (after the droplet splitting K-channel) enables integrated washing of magnetic beads within rapidly-moving droplets. Ultimately, the K-channel provides an exciting opportunity to perform many useful droplet operations across a range of magnitudes without requiring architectural modifications. Therefore, we envision the K-channel as a versatile, easy to use microfluidic component enabling diverse, in-droplet (bio)chemical manipulations.
Congrats to Alex on Her Recent Publication
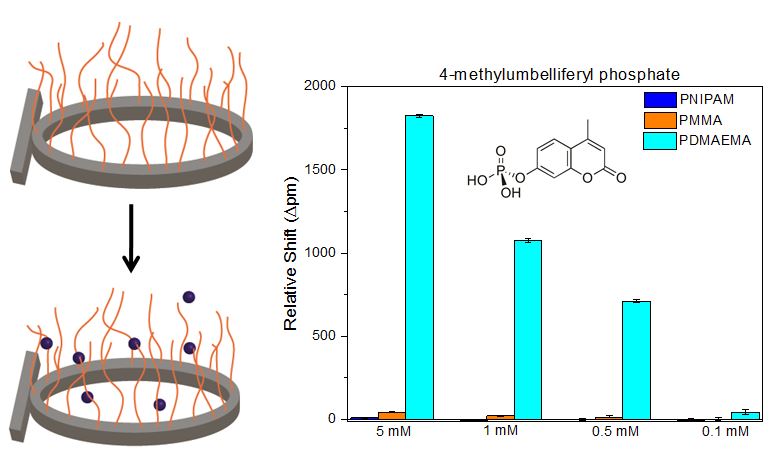
Polymer Brush-Modified Microring Resonators for Partition-Enhanced Small Molecule Chemical Detection, has been accepted for publication in Wiley-VCH's newest journal, ChemistrySelect. This article is the result of a collaboration with Paul Braun's Materials Science and Engineering group at the University of Illinois at Urbana-Champaign and introduces the idea of organically modifying our ring resonators for specific and selective detection.
Congrats to Dan on his most recent paper published in Langmuir!
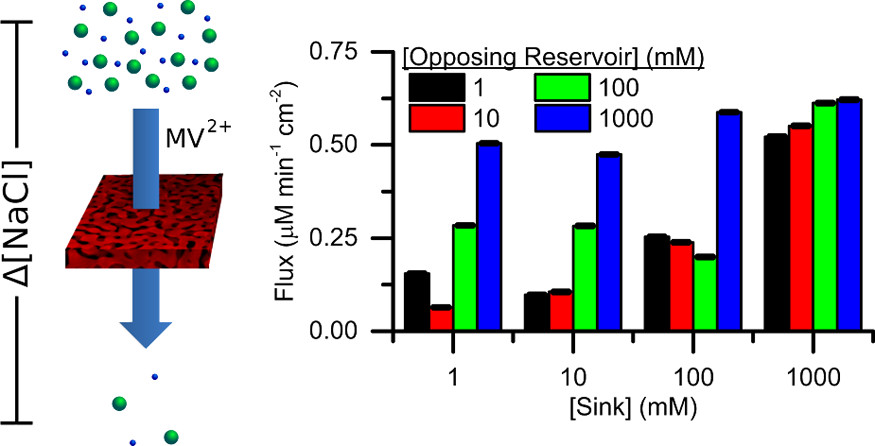
Dan's most recent paper published in Langmuir. This is an extension of his previous work published last year, which demonstrated nanoporous gold (NPG) as a material with selective and tunable chemical transport properties.
Electrolyte Gradient-Based Modulation of Molecular Transport through Nanoporous Gold Membranes
Abstract
Nanopores, and nanoporous materials in general, are interesting for applications in chemical and biomolecular transport as pore sizes are on the same scale as the dimension of many (bio)chemical species. Many studies have focused on either single pores or small arrays of cylindrical pores, which are convenient in terms of their amenability toward computational modeling of transport phenomenon. However, the limited overall porosity may inhibit transport flux as well as the eventual implementation of these materials as active separation elements. Inspired by its relatively high porosity, we have explored nanoporous gold (NPG) as a membrane across which small molecular species can be transported. NPG offers a random, bicontinuous pore geometry, while also being inherently conductive and readily amenable to surface modification—attributes that may be enabling in the pursuit of size- and charge-based approaches to molecular separations. NPG was fabricated via a free-corrosion process whereby immersion of Au-containing alloys in concentrated nitric acid preferentially dissolves the less noble metals (e.g., Ni, Cu). Average pore diameters of 50 ± 20 nm were obtained as verified under scanning electron microscopy. NPG membranes were sandwiched between two reservoirs, and the selective transport of chemical species across the membrane in the presence of an ionic strength gradient was investigated. The flux of small molecules were monitored by UV–vis absorption spectrometry and found to be dependent upon the direction and magnitude of the ionic strength gradient. Moreover, transport trends underscored the effects of surface charge in a confined environment, considering that the pore diameters were on the same scale as the electrical double layer experienced by molecules transiting the membrane. Under such conditions, the transport of anions and cations through NPG was found to depend on an induced electric field as well as ion advection. Further electrical and surface chemical modulations of transport are expected to engender increased membrane functionality.
Alex Receives a Travel Award for ACS
Alex was one of three students awarded the Research Corporation Physical Chemistry Travel Award. This award supports travel to the 253rd American Chemical Society National Meeting in San Francisco, CA this spring, where Alex will give a talk "Facile in-situ polymer brush characterization using whispering gallery mode sensors."
Mad Minute with Ryan Bailey
The Michigan Center for Integrative Research in Clinical Care (MCIRCC) profiled Ryan as part of their "Mad Minute" series. Click here to read about our lab's efforts related to clinical and to read more about MCIRCC.
Cover of First C&EN of 2017 Highlights the Lab's Work
Ryan joins Analyst Editorial Board
New year, new editorial duties! Ryan has joined the Editorial Board of Analyst as as Associate Editor. As you might expect from the journal title, Analyst published high quality analytical research that fall under three broad scopes: bioanalytical, analytical nanoscience, and advanced analytical systems.
The lab has published three manuscripts in the journal including:
- Multiplexed evaluation of capture agent binding kinetics using arrays of silicon photonic microring resonators, J.-Y. Byeon, R.C. Bailey, Analyst, 2011, 136, 3430-3433.
DOI: 10.1039/c0an00853b - Photonics-on-a-Chip: Integrated Waveguides as Enabling Detection Elements for Lab-on-a-Chip Biosensing Applications, A.L. Washburn and R.C. Bailey, Analyst, 2011, 136, 227-236.
DOI: 10.1039/C0AN00449A - Multiplexed Cancer Biomarker Detection Using Chip-Integrated Silicon Photonic Sensor Arrays, A.L. Washburn, W.W. Shia, K.A. Lenkeit, S.-H. Lee, R.C. Bailey Analyst, 2016, 141, 5358-5365
DOI: 10.1039/C6AN01076H - Featured on the Cover of the Journal
Congratulations to Ryan!
ACS Central Science Highlights Technology from the Lab
Here's a snippet from the article:
Adding more tests to an instrument will necessitate new detection strategies that don’t require separating the sample into ever-tinier aliquots for each test. One promising approach came from Ryan C. Bailey, now at the University of Michigan, and Genalyte, a company he helped launch in 2007 to explore the blood-testing potential of a technology based on silicon microring resonators.
When light circulates in a cavity, the light waves that are in phase resonate, amplifying the signal. It’s the optical equivalent of rubbing a finger on the rim of a wine glass to create a resonant sound wave. Altering the volume of wine in the glass changes the pitch of the note. This concept led Bailey and his co-workers to carve picometer-wide rings into silicon chips that they stud with antibodies to blood components like proteins, nucleic acid disease markers, or pathogens. When the antibodies bind to antigens like these in a blood sample, the bound molecules change the refractive index of each ring’s resonating light and, thus, the color of the amplified light.
The technique is sensitive and inexpensive, but what is most promising about the technology to Bailey is that it can detect many antigens at a time. Genalyte’s chips each currently hold 128 rings, and a 250 μL sample “goes over all sensors in one shot”, says CEO Gunn. Clinical studies by the company show that capillary blood works as well as venous blood with the chips.
Their first target for commercialization is a chip including the 20 or so protein tests that a rheumatologist would commonly order to help diagnose autoimmune diseases such as lupus; the company is working on getting FDA approval for this device.